Though I am not a photographer by trade, I photograph every instance of iron films that I happen across. I greatly enjoy capturing the incredible diversity of these films in nature; no two films are alike! This page will include nearly all of my photos and will be arranged in chronological order. All locations will be appropriately labeled wth the date and location.
Shaver's Creek, PA
October 3rd, 2022




I was revisiting Shaver's Creek with a class, and some young silver films turned up. I had to do the awkward thing and squat down to take pictures of them even when the class was meant to be doing something else. One of my classmates asked "Oh, are you looking for iron films?".
Bruebaker Run, PA
August 28th, 2022










A classmate and I revisted Bruebaker Run on this sticky hot August day to obtain sediment and water innocula for a set of Winogradsky columns. I was able to take some wonderful pictures of films at the effluence site. I just love how the vivid color of the iron-rich sediment looks underneath the vibrant hues on film surfaces. We also took a bit of a walk (which was not pleasant due to the temperature, unfortunately) and got to see some (possibly defunct?) treatment ponds. One of these was filled with these strange-looking floating mats in crystal-clear water. The sight was so alien to me that it looked like something out of a sci-fi novel.
Cutler Coast, ME
August 10th, 2022











Our next stop was a ~10 mile loop hike on the Cutler Coast, which featured a 5 mile hike out to Fairy Head through the woods and a ~5 mile hike back along the coast. We stopped for a bit at Fairy Head to investigate the rock formations and the wonderfully dark humic-acid rich freshwater pools that dotted the landscape. At some point along our hike along the coast, I finally found what I was looking for - films! These films were fed by a freshwater trickle that ran down and pooled inside some rock crevasses.
Great Wass Island, ME
August 9th, 2022
















Upon our visit to Great Wass Island we went on a ~4 mile hike around the Island's perimeter. Not only was it exceedingly beautiful, and for us to enjoy almost entirely by ourselves, but there were films!
Before this day I had only seen iron films in areas with a saltwater influence extremely rarely, but here at the Atlantic shoreline were films, unmistakably! In my excitement I went as far as to taste the water for salt to check if we were dealing with real oceanic tide pools or fresh groundwater seeps. My palate let me know that it was most definitely salt.
I used to think that iron films could only be made by a single species of bacteria - that being Leptothrix discophora. But L. discophora is a freshwater bacterium. This sighting made me start thinking more deeply about how films are formed in nature.
Cobscook Bay, ME
August 8th, 2022






That same friend (from the last entry) and I took a trip to the Eastern point of Maine to camp at Cobscook Bay for a week. As always, I was on the lookout for films. After settling down in the campsite we took to the shoreline of the bay and I noticed some shiny patches. The small size of these films made them blend in with the regular beach debris quite well.
Mansfield Hollow, CT
July 17th, 2022












I visited my friend at UCONN around this time, and we took a morning walk at a nearby resevoir at Mansfield Hollow. The muddy, footprint-indented shoreline of the resevoir provided perfect shelter from the wind for the formation of some wonderful films. I always delight in seeing films form in muddy environments, because the dissonance between the rather "visually unappealing" mud versus the vibrant rainbow hues of the iron films is quite fun.
Bruebaker Run, PA
July 12th, 2022
























Bruebaker Run is a similar case to the Beaver Run borehole, though the water that flows out of this effluence is definitively acid mine drainage. The area is scarred, the acidic water having killed a good number of trees and plants, and the decimation can be observed on Google Maps as an orange stain on the landscape. I find this location hauntingly beautiful, and it produces some of the most vibrant yet oddly transparent films I have ever had the pleasure of viewing.
The water here flows out from an ominous hole in the ground, bubbling up over its limits and then spreading out in a fan of shallow streams that flows swiftly downhill and into Bruebaker Run. The sediment is water-saturated and muddy, making it somewhat hard to traverse, but also features terrace-like formations that seem encrusted with minerals (possibly iron minerals). In areas where this drainage water is sent in ruffles downstream, the mini-rapids that are created are teeming with unusually bulbous-shaped green growths, which may be algal colonies.
Though the harm that acid mine drainage inflicts on the environment and health of locals in areas which are affected cannot be overstated, I find locations like this fascinating. Even in landscapes that cannot host forms of life that are familiar to us, like trees, these areas are actually bursting at the seams with microbial diversity - and films!
Beaver Run Borehole, PA
June 10th, 2022












Central Pennsylvania is riddled with abandoned coal mine shafts. This borehole was, as I've been told, meant to act as a vent to prevent the buildup of gasses in one such mine. The mine has since flooded, however, and thus what was meant as a gas vent is now acting as more of a spring.
The sulfur-rich water that bubbles up from the abandoned coal mine beneath the borehole feeds filamentous sulfur oxiding bacteria in the stream, including Thiothrix, which grow to form feathery "sulfur streamers" which wave back and forth in the stream flow like some kind of alien plant (though these bacteria are natural, and are by no means closely related to any type of plant). Though this effluence is an instance of mine drainage, the borehole water is actually circumneutral in nature - e.g. not acidic. This has led some of my coworkers to call it "circumneutral mine drainage", which, while accurate, I find slightly humorous. It has me wondering what "basic mine drainage" would look like, but nothing comes up when I google it.
Shaver's Creek, PA
October 9th, 2021



This location, nearby to the synonymous Shaver's Creek Environmental Center, involves a short walk through some lakeside woods to the point where Shaver's Creek outlets into Lake Perez. The flow rate of Shaver's Creek was slow on this date, leaving ample opportunity for iron films to spread over the surface. My only regret is that I didn't get down through the brush to take some close-ups. I've revisited this site a few times, but haven't seen the amazing film blanket phenomenon that I saw on this day.
Enders Falls, CT
July 24th, 2021

No films on this day - indeed, I don't think I've ever seen films at Ender's Falls. It's likely that the flow rate is too fast. However, I thought this image was rather stunning, and so wanted to include it in the gallery. The humic-rich amber color of the water at Ender's Falls has always fascinated me.
Showing off my slide collection here. At some point during my undergraduate thesis I realized that I might be able to sample films by using microscope slides, and so I ordered some oversized microscope slides to work with. What started as a bit of a game to capture the best photographs I could of films turned into something rather resembling a trading card collection, except that in this type of trading card game, all of the cards are holographic ultra rares!
Rust Brook (Hawley Brook), CT
May 7th, 2021



Though part of the appeal of hunting for iron films to photograph is to find beautiful specimens, I think the dull ones deserve recognition as well.
Buckingham Seep (Hawley Brook), CT
January 11, 2021



















Buckingham Seep is the name I have given to a series of of small iron-rich groundwater seeps near the Rust Brook effluence in the nearby Buckingham park. This day, January 11th, was the first time that I had discovered the site and so I eagerly photographed all that I could. The films were exceptionally thick, richly patterned, and vibrant. During this trip I noticed a number of groundwater sampling stations. It appears that someone, at some point, was aware that the groundwater might warrant sampling.

Films aren't always stunning, but they are always exciting to see no matter what!
Kameoka, Japan
March 3, 2020




Some pictures I took of iron-rich drainage that I found after taking a ride on the Sagano Romantic Train. Most of the iron films that I see in the U.S. are in effluences affected by acid mine drainage, where large areas are contaminated with mine wastewater - or in iron seeps, where iron-rich groundwater comes to the surface. In Japan, however, it was mostly in small artificial drainage areas.
Takaragaike Park, Kyoto, Japan
February 24, 2020













I had the amazing opportunity to study abroad in Kyoto during the Spring semester of my junior year. Though ultimately the trip was unfortunately timed and cut short due to the spread of COVID-19, I had two wonderful months at Doushisha University and with my host family.
Potentially unsurprisingly, I also saw a good amount of films while I was in Japan, most of them being in artificial drainage pipes, waterways, and the like. Some of these films ended up being particularly vivid, and I am very proud of these photos.
Photographing water pollution in random urban gullies, however, got me a few looks. On one occassion, while taking a picture of some iron films on the side of the road, I heard a passerby say to his friend (translated to the best of my meagre ability, of course), "tourists will just take pictures of anything".
Fushimi Inari, Kyoto, Japan
February 8, 2020





No films here, but some iron-rich flocculate deposits that I saw at the Fushimi Inari shrine. Even when trying to do touristy things, I ended up taking pictures of iron film and flocculate.
Rokujizo, Kyoto, Japan
January 25, 2020





Some of the first films that I saw on my travels in Japan. These thin silver films were in a small farm plot in the suburbia surrounding Kyoto, Japan. I often find that in areas without significant amounts of visible iron oxides (e.g. orange sediments, slicks, or flocculates), any films that form are more than likely to be silver ones. Silver, being the first color that thin-film interference optics produces, means that the films are only a few nanometers thin.
Oberlin, OH
October 9, 2019



I attended Oberlin College as an undergraduate, which is where I learned about Leptothix discophora and iron films in general. Surprisingly (or not!) I also encountered a few films on campus.
Pictured Rocks, MI
August 10, 2019





During the Summer of 2019, I took a break from my field station job to travel to the Upper Peninsula of Michigan with some of my friends and coworkers. The main event of the trip was an 11 mile loop hike at Pictured Rocks National Lakeshore, which was an absolutely stunning natural location. I took more than a hundred pictures - some of which were iron films!
Trout Lake Station, WI
August 8, 2019








Not to be mistaken for iron films, the following are images of other biofilms that formed inside of a series of "biological batteries" that I constructed as part of an experiment during my REU at Trout Lake Station in the summer of 2019. Note the "roy g biv" color ordination - the biofilm colors in these images proceed in the order of the rainbow. These films are not examples of thin-film interference, which is the optical phenomenon by which iron films get their characteristic hues. Though both might look at a glance to be "rainbows", the thin-film spectrum does not proceed in rainbow order, as seen here.
Additionally, you may notice that the "coloration" of these films is radial in nature. This is a consequence of the photographing method, as the iridescent effects of these films was only visible when a bright light was shone on the film. In contrast, thin-film intereference effects, as seen in iron films, are inherent to a specific section of film due to the fact that thin-film color effects depend on the thickness of a film in any one location. In these images, the "rainbow" effect moved alongside the flash of the camera, while the blues and hues of a true iron film would remain in place when the angle of light is shifted - though they may be visible or invisible from certain angles. This is a result of these "iridescnent" effects being due to diffraction as opposed to thin film interference like iron films.
Bond Falls, MI
August 4, 2019






During the summer of 2019, I was employed as an REU intern at Trout Lake Station, WI. Though most of my days were spent doing early-morning field monitoring, me and the other interns at the station were able to plan a few trips. One of these was to Bond Falls in the upper peninsula. Though I didn't expect to see any films here due to the nature of the site (films and fast flowing water don't mix, after all) I did see a few.
Trout Bog and North and South Sparkling Bogs, WI
May 27, 2019

























Over the course of summer 2019, I worked as an intern at Trout Lake Station in the northwoods of Wisconsin. My responsibilities included weekly field monitoring, which entailed that I travel to a series of bogs in the area and take physical depth profile measurements (temperature, dissolved oxygen, pH, etc).
While I was certainly expecting to encounter my fair share of bacteria at the bogs, including Chlorobi , I was not expecting to see surface water films. Even more surprisingly, I was not prepared to watch them form before my eyes in mere seconds - acting wholly unlike the films I had grown to love. These films sprung to the surface of the water from disturbed puddles and bog shoreline, forming strange and liquid-like patterns on the surface of the water. They were vibrant, pitted with holes, and seemed to bubble up from the depths. I found myself counfounded in that moment - here I was seeing films, and confusing them with oil. The films swirled, moved, and formed like fluid. And yet they broke, shattering cleanly at their edges.
I still do not know what these films are. I have not seen them since, and they are (to the best of my knowledge) completely undocumented in the literature or elsewhere. The two photos I have found online ( here / here )that resemble these that I saw at Trout Bog refer to them as iron films. Yet there are many physical features that make them distinct, which I hope to outline in a future article. I hope to encounter these films again someday so that I can sample them and analyze what they are made of. I find it likely that these films may be organic in nature, possibly originating from the decomposition of organic matter.
Appledore Island, ME
July 27, 2018











Central Pond, so named for its location, is a shallow brackish-water pond sandwiched in a low-lying dike between the upper and lower lobes of Appledore island. Brackish in nature, Central Pond is fed primarily by a combination of fresh rainwater and incoming ocean waves which are able to breach the short distance between the shore and the pond during storms. Between precipitation events, water held in the pond will slowly evaporate, leading to a fluctuating water level. Because no rivers or streams flow into the pond, the water surface is often still, making it a great place for iron films to form. Anecdotally, the pond is popular with birds on the island!
I was able to witness this wonderful display of iron films in Central Pond towards the end of my stay at Appledore Island for a field science course. These videos were taken shortly before a storm, hence the somewhat dull lighting.
Rust Brook (Hawley Brook), CT
June 30, 2018











Rust Brook (Hawley Brook), CT
June 12, 2018









































































































































































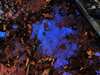





































Rust Brook is the name that I have chosen for a 100-meter long effluence that discharges into Hawley Brook, a short stream in the larger Farmington River watershed. Hawley Brook originates in a low-lying, marshy area within Found Land Park in central Connecticut, where assumedly it is fed via runoff from the surrounding higher-altitude land encircling it. From there, the brook flows through the forest, under Huckleberry Hill Road, and then into Countryside Park where it feeds two resevoirs. From here, the water continues on to ultimately discharge into the Farmington River.
Rust Brook, in contrast, is a man-made drainage effluence. While the beginning of the Rust Brook is inset into its surrounding area, with relatively defined banks, the stream significantly spreads out the further downstream you go. Where the brook discharges into Hawley it is more of a fan of waterlogged rusty-red soil as opposed to having any sort of streambank.
As one can tell from looking at the photos below, Rust Brook was named for its high iron content and rusty-red appearance. While the water in Rust Brook is not actually red, the bottom of the stream it coated in a thick layer of iron precipitates.
Iron films, such as the ones that I've photographed on this trip, are indeed natural phenomenon, but it is likely that Rust Brook itself is an instance of acid mine drainage (AMD) - though in this case it may also be referred to as acid rock drainage (ARD) because there is no mining operation involved.
The likely origin of Rust Brook's source of water (and potentially contamination) is from Buckingham transfer station, which is the site of an old decomissioned dump that used to be active until the late 1970's. Surrounding the dump is a dug out drainage ditch that usually runs dry, but on rainy days will fill with runoff from the dump and drain into a pipe, which then travels under Huckleberry Hill Road and presumably ends where Rust Brook begins, in Found Land park. However it is interesting to note that there is no charcteristic iron precipates on the dump-side of the pipe. Only after the water enters into the pipe and out the other side does the iron seem to precipitate out. In fact the dump side is often dry (not even a trickle) meanwhile Rust Brook continues to run steadily even during longer periods without rain. This gives me the impression that there is another water source feeding the brook - one that is unknown to me. I wonder if the iron is coming from the dump, or from somewhere else ...
Farmington River, CT
June 21, 2018






This shiny blue color is a common appearance of very thin iron films - in fact, the films are usually a silver hue in color, but reflect the sky above them like a mirror. On a sunny blue day, this imparts them with a reflective blue color.
The Farmington River has never been a good location for me to look for films. It's fast-flowing nature and steep banks tend to prevent films from forming; even these that managed to form had to do so in the shelter of puddles along the rocky shores.
Sometimes, iron films seem to form on mud. It's my guess that they are actually forming on surface water, per usual, which then subsides due to a lowering of the water level (e.g. days after a rain initially causes an increase in water level). Once the surface dries up completely, though, the films seem to disappear.
Vermilion River, OH
May 27, 2018









During the Fall of 2017, I took an introductory writing seminar at Oberlin College that was about living in the Vermilion River watershed. The course concerned the natural history of the Vermilion river and the land surrounding it, starting with molten rock and glacial till and continuing to the present. As part of this course, we were visited by the photographer Linda Grashoff, who intoduced us to the phenomenon of iron films and the bacteria Leptothrix discophorawho may play a role in causing them. I was taken by the wonderful coloration of these films and subsequently began noticing them everywhere. My fascination with iron films has (evidently) continued to this day.
For more pictures of iron films in the Vermilion River watershed, please visit photographer Linda Grashoff's wonderful blog: Romancing Reality. I owe my current interest in iron films to her photography and book They Breathe Iron.
These are the first iron film photos that I ever took (on a class tripto the Chance Creek Nature Preserve), so I hold them dear to my heart.